Atlanta-based Guided Therapeutics has shipped five of its LuViva Advanced Cervical Scan devices to Shandong Yaohua Medical Device Technology. The scanners will undergo lab testing and clinical trials aimed at winning approval by the Chinese Food and Drug Administration (CFDA).
Shandong Yaohua will begin testing LuViva in clinical trials, and expects to apply for CFDA approval before the year’s end. Upon approval, the devices will go on sale early next year in China, which is potentially the world’s largest market for cervical cancer screening. China has the world’s highest incidence of the disease, and more than 390 million Chinese women are in the recommended ages for screening.
 Shandong Medical will pay all expenses related to testing LuViva. It will also submit results to CFDA as well as distribute and commercialize the device within China.
Shandong Medical will pay all expenses related to testing LuViva. It will also submit results to CFDA as well as distribute and commercialize the device within China.
“We are gratified that the level of cooperation between our two companies has allowed us to make significant progress toward entering the Chinese market with LuViva,” Gene Cartwright, CEO and president of Guided Therapeutics, said in a news release.
Added Li Yaohua, chairman of Shandong Yaohua: “We are happy that our plan for bringing LuViva to China has gotten off to a good start and are optimistic regarding the future success of this important program, which we believe will improve the healthcare of women by the earlier detection of cervical cancer, when it is most treatable.”
Shandong Yaohua is located in the high-tech development zone of Shandong province, where it owns an 86,600-square-meter production facility with 20,000 square meters of floor space for assembly of medical devices. The company’s 2015 revenues came to $12.7 million.
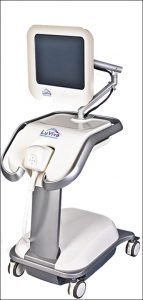
Cytology (Pap test) is currently China’s most widely used method for primary cervical cancer screening and diagnosis. LuViva is a technologically advanced, non-invasive diagnostic device that scans the cervix with light and uses spectroscopy to measure how light interacts with the cervical tissue. Guided Therapeutics says LuViva reduces the overall cost of cervical cancer diagnosis through the elimination of many unnecessary colposcopies and biopsies by identifying false positives.
In addition, the device has shown to detect disease missed by colposcopy and biopsy. Early detection not only reduces patient morbidity, but also costs for the healthcare system.

